Abstract
Introduction: Malignancy-associated hemophagocytic lymphohistiocytosis (M-HLH) is a rare, life-threatening condition characterized by profound immune dysregulation (Tamamyan Cancer, 2016). The management of M-HLH is highly individualized and generally consists of treatment directed at the malignancy itself with or without HLH-specific therapy. Few small series exist to describe clinical outcomes and guide management beyond consensus guidelines (La Rosée Blood, 2019). Emapalumab is a humanized monoclonal antibody targeting interferon-gamma (INF-γ) and is FDA-approved for treating primary HLH in adults and children relapsing after or intolerant to conventional therapy (Locatelli NEJM, 2020). However, to our knowledge, there are no existing data describing the outcomes of patients with M-HLH treated with emapalumab.
Methods: We queried the Memorial Sloan Kettering Cancer Center database to identify adult patients with M-HLH who received at least one dose of emapalumab. Their medical records were reviewed, and baseline clinical characteristics, vital signs, and laboratory results were extracted from the initiation of emapalumab until death or last follow-up. HLH-2004 criteria were determined as previously described (Henter Ped Blood Cancer, 2007). Response criteria were adapted from the published prospective emapalumab trial (Locatelli NEJM, 2020). Median percent change in HLH-biomarkers and overall survival were calculated from the initiation of emapalumab until death or last follow-up. Statistical analysis was performed using GraphPad Prism V9.1. This study was approved by our institutional IRB.
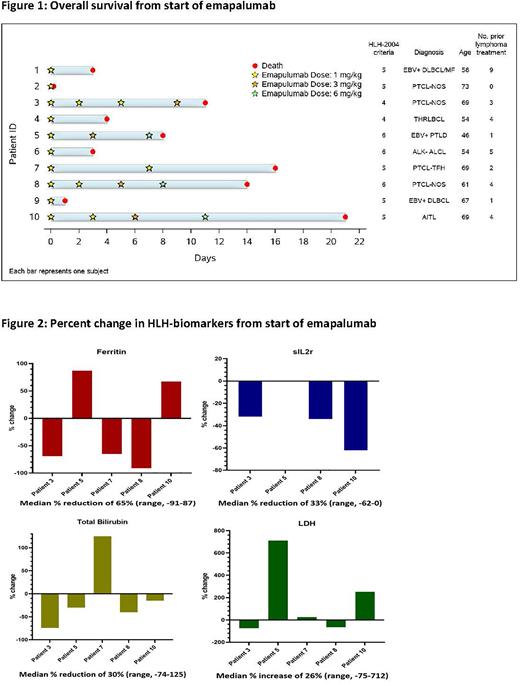
Results: We identified ten patients. The median age was 64 years (range, 46-73). All patients had active cancer at the time of M-HLH diagnosis and all were admitted during treatment. Six patients had peripheral T-cell lymphoma, three patients had large B-cell lymphoma (three of which were positive for Epstein-Barr Virus (EBV), and one patient had an EBV+ post-transplant lymphoproliferative disorder. All but one patient had relapsed or refractory disease and the median number of prior lines of therapy was 3.5 (range, 0-9). All patients were receiving high-dose steroids prior to and concurrently with emapalumab, and all but one patient received etoposide prior to emapalumab. Eight patients had an HLH-2004 score of five or higher while two patients had a score of four.
Regarding disease manifestations, all patients had cytopenias as a predominant feature; all patients had either elevated total bilirubin or elevated transaminases; six patients had clinical evidence of CNS involvement, and three patients had acute renal failure. The median number of emapalumab doses received was 1.5 (range, 1-4). The median survival was 6 days (range, 1-21) (Figure 1).
Five patients survived for >72 hours and received more than one emapalumab dose. These patients were evaluated for response (Figure 2). The median percent reduction in ferritin was 65% (range, -91 to 87%) with a ≥50% reduction in three patients. The median percent reduction in soluble IL-2 receptor α (sIL2r) was 33% (range, 0 to -62%) with a ≥50% reduction in one patient. The median percent reduction in total bilirubin was 30% (range, -74 to 125%) with a ≥50% reduction in one patient. The median percent increase in LDH was 26% (range, -75 to 712%). One patient was treated while requiring vasopressor support and high-flow nasal cannula and was subsequently weaned off pressors and supplemental oxygen. Two patients showed a ≥50% reduction in HLH-biomarkers or ≥50% organ improvement in ≥3 baseline abnormalities. These patients were considered to have improvement in HLH by the previously described response criteria. No patient had a complete response.
Conclusions: Overall survival was poor in these ten heavily pre-treated patients with M-HLH who received emapalumab. However, four out of the five patients who received more than one dose showed sustained improvement in at least one HLH-biomarker and/or organ function suggesting inhibition of INF-γ maybe a reputable target to aid in mitigating the cytokine dysregulation of M-HLH. Additional preclinical and clinical work utilizing this therapy in treating M-HLH and other forms of secondary HLH are needed. In particular, if combination therapy used earlier in the course with other agents such as etoposide, ruxolitinib, or disease-specific therapy may improve outcomes merits further investigation.
Disclosures
Johnson:Myeloid Therapeutics: Membership on an entity's Board of Directors or advisory committees. Salles:Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria; AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees. Moskowitz:Miragen: Research Funding; Imbrium Therapeutics L.P./Purdue: Honoraria; Takeda: Honoraria; SecuraBio: Research Funding; Affimed: Honoraria; ADC Therapeutics: Research Funding; Seattle Genetics: Honoraria; Merck: Research Funding; Bristol-Myers Squibb: Research Funding; Janpix Ltd: Honoraria; Merck: Honoraria; Seattle Genetics: Research Funding; Incyte: Research Funding; Biegene: Research Funding. Vardhana:Immunai: Membership on an entity's Board of Directors or advisory committees; Koch Disruptive Technologies: Consultancy. Horwitz:Verastem/SecuraBio: Research Funding; Seattle Genetics,: Research Funding; C4: Research Funding; Takeda: Consultancy; ADC Therapeutics: Research Funding; Celgene: Research Funding; Shoreline Biosciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Yingli Pharma Limited and Tubulis: Honoraria; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Affimed: Research Funding; Kyowa Hakko Kirin: Research Funding; Daiichi Sankyo: Research Funding; Millennium /Takeda: Research Funding; Kyowa Hakko Kirin: Consultancy; SecuraBio: Honoraria; Crispr Therapeutics: Research Funding; ONO Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Affimed,: Consultancy; Cimieo Therapeutics: Honoraria.
OffLabel Disclosure:
Emapalumab is not currently approved for secondary Hemophagocytic Lymphohistiocytosis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal